Interestingly two major reports get published for the long weekend read.
Remdesivir report – https://www.nejm.org/doi/full/10.1056/NEJMoa2007764
The conclusion as stated by the report:
“Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19 and evidence of lower respiratory tract infection.”
Key points in the study not highlighted but are key takaways
“We conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with Covid-19 with evidence of lower respiratory tract involvement.”
Wow – a real study….no back casting statistical magic! Incredible it can be done so quick…
“Serious adverse events were reported for 114 of the 541 patients in the remdesivir group who underwent randomization (21.1%) and 141 of the 522 patients in the placebo group who underwent randomization (27.0%).”
“These preliminary findings support the use of remdesivir for patients who are hospitalized with Covid-19 and require supplemental oxygen therapy. However, given high mortality despite the use of remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient.”
Author affiliation section – “Gilead Sciences,”
HCQ report – https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext
Conclusion as stated by the report:
“In summary, this multinational, observational, real-world study of patients with COVID-19 requiring hospitalisation found that the use of a regimen containing hydroxychloroquine or chloroquine (with or without a macrolide) was associated with no evidence of benefit, but instead was associated with an increase in the risk of ventricular arrhythmias and a greater hazard for in-hospital death with COVID-19.”
Some takeaways immediately from the summary – NOT a study – another math game.
FYI Macrolide: One in a class of antibiotics that includes Biaxin (Clarithromycin), Zithromax (Azithromycin), Dificid (Fidoximycin), and Erythromycin. The macrolides inhibit the growth of bacteria and are often prescribed to treat rather common bacterial infections.
Two major objectives of the study:
“The primary outcome of interest was the association between use of a treatment regimen containing chloroquine or hydroxychloroquine (with or without a second-generation macrolide) when initiated early after COVID-19 diagnosis with the endpoint of in-hospital mortality. The secondary outcome of interest was the association between these treatment regimens and the occurrence of clinically significant ventricular arrhythmias (defined as the first occurrence of a non-sustained [at least 6 sec] or sustained ventricular tachycardia or ventricular fibrillation) during hospitalisation.”
Still no Zinc combo in the study even though this is what has seemed to be prescribed to those promoting it. And we do have a science journal showing the HCQ ability to allow for better absorption ionic zinc.
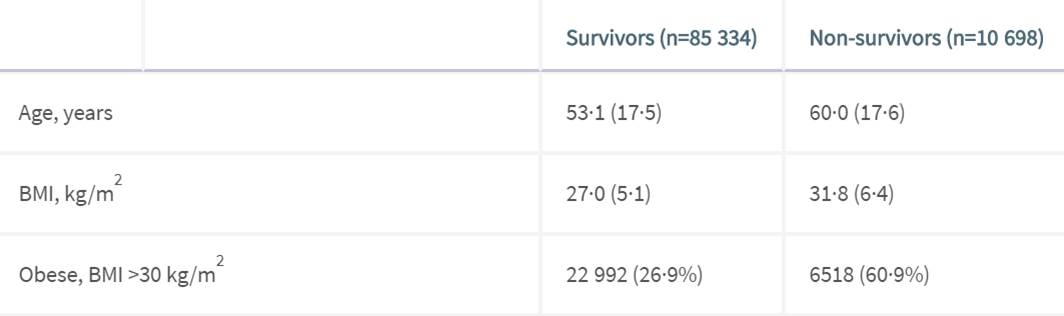
Table 1 shows a list of survivors and non survivors and the corresponding stat and %. IN GENERAL the two columns are close except for the following
DON’T BE OBESE – 60.9% of non survivors are obese.
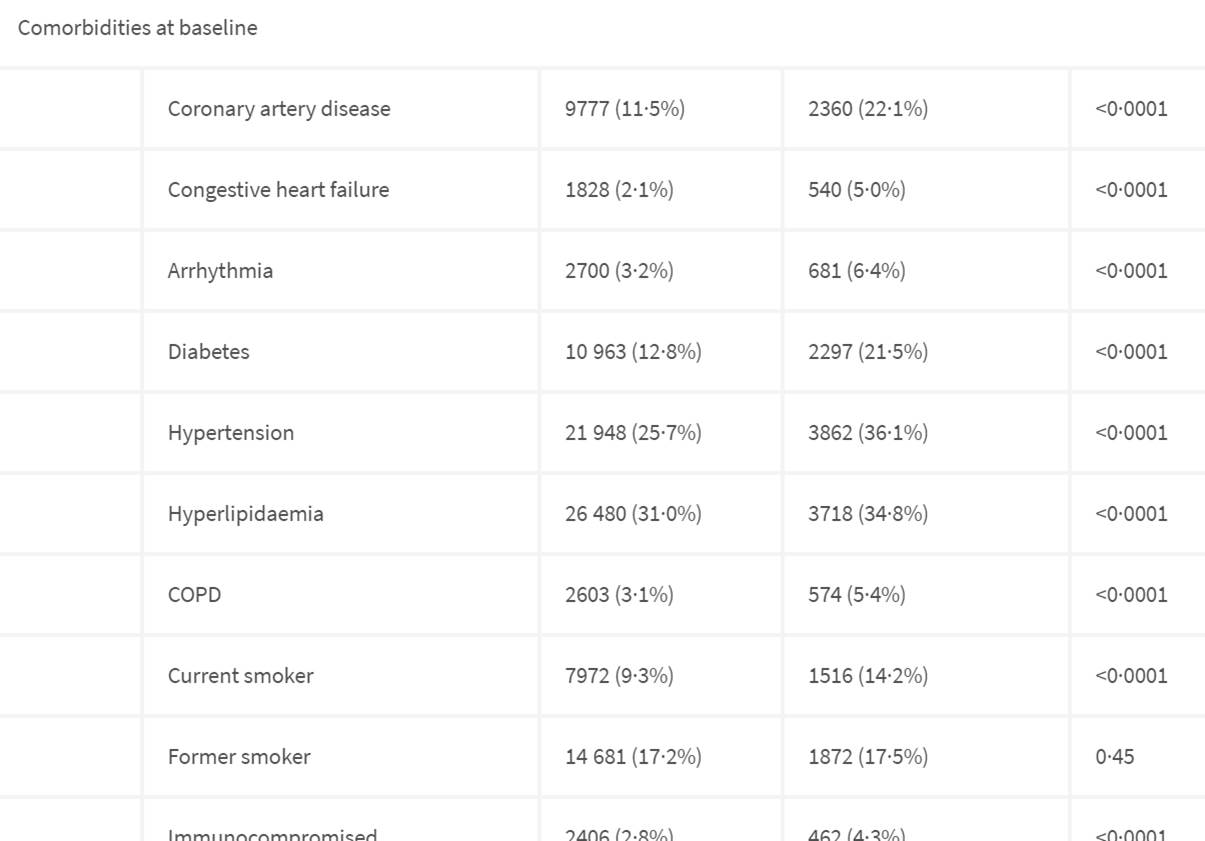
Heart disease, diabetes,hypertension are other issues to concern yourself.
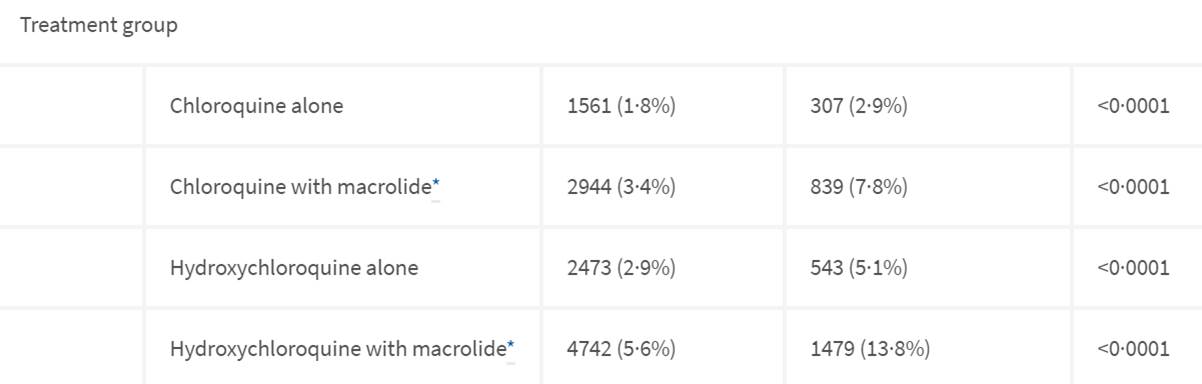
This part of table 1 is very interesting which I presume is where they are drawing the conclusion of HCQ failed
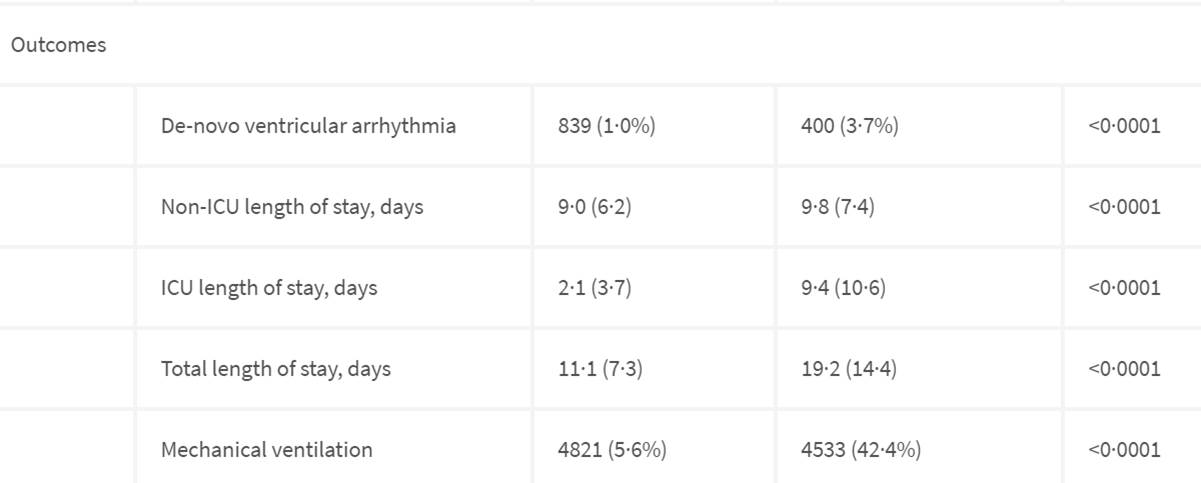
And outcomes show don’t get on ventilator….
Having this ventricular arrhthmia is associated 3.7% of those not surviving! This is the big second conclusion? the concern of the 3.7% of the non surviving group? This is the side effect that HCQ could cause.
Once again 53 Million+ people have taken HCQ – here is the advisory report from CDC to recommend those going to certain countries to take HCQ — https://www.cdc.gov/malaria/resources/pdf/fsp/drugs/Hydroxychloroquine.pdf No mentioned of heart issues – now perhaps the extension is with this particular virus with HCQ is causing this adverse reaction this perhaps could be the concern – but 3.7% in non survivor category and 1% in survivor category I would think there are bigger adverse reactions to concern about – note remdesivir 21% serious adverse reaction.
I do think the authors did a good job stating the limitations of the study but those who want to see a certain thing will not even note the limitations.
“Our study has several limitations. The association of decreased survival with hydroxychloroquine or chloroquine treatment regimens should be interpreted cautiously. Due to the observational study design, we cannot exclude the possibility of unmeasured confounding factors, although we have reassuringly noted consistency between the primary analysis and the propensity score matched analyses. Nevertheless, a cause-and-effect relationship between drug therapy and survival should not be inferred. These data do not apply to the use of any treatment regimen used in the ambulatory, out-of-hospital setting. Randomised clinical trials will be required before any conclusion can be reached regarding benefit or harm of these agents in COVID-19 patients. We also note that although we evaluated the relationship of the drug treatment regimens with the occurrence of ventricular arrhythmias, we did not measure QT intervals, nor did we stratify the arrhythmia pattern (such as torsade de pointes). We also did not establish if the association of increased risk of in-hospital death with use of the drug regimens is linked directly to their cardiovascular risk, nor did we conduct a drug dose-response analysis of the observed risks. Even if these limitations suggest a conservative interpretation of the findings, we believe that the absence of any observed benefit could still represent a reasonable explanation.”
On the other side of the world in India we have – https://theprint.in/health/hcq-breakthrough-icmr-finds-its-effective-in-preventing-coronavirus-expands-its-use/427583/?
“Three studies find that hydroxychloroquine reduces chances of contracting Covid, so ICMR allows more frontline workers to take it as a preventive drug.”
“National Institute of Virology in Pune has found in laboratory testing that HCQ reduces the viral load.”
“ICMR also analysed data collected previously, known as retrospective case-control analysis, and found “a significant” relationship between “the number of doses taken and frequency of occurrence of Covid-19 infection in symptomatic healthcare workers who were tested for SARS-CoV-2 infection”.”
“Another observational study was conducted among 334 healthcare workers at the country’s largest public hospital, New Delhi’s All India Institute of Medical Sciences (AIIMS). The 248 workers who took HCQ as preventive drug for an average of six weeks had lower incidence of the infection than those not taking the pill.”
“The ICMR had earlier announced that some side effects, such as abdominal pain and nausea, have been observed in healthcare workers who were administered HCQ.”
As noted by the CDC!
“The anti-malaria drug is often blamed for triggering irregular heartbeat.
However, in the final results of the studies (HCQ prophylaxis among 1,323 healthcare workers), the ICMR found mild adverse effects such as nausea in 8.9 per cent workers, abdominal pain in 7.3 per cent, vomiting in 1.5 per cent, low blood sugar (hypoglycaemia) in 1.7 per cent and cardio-vascular effects in 1.9 per cent.
The advisory states the drug should be discontinued if it causes the “rare” side effects related to the heart, such as cardiomyopathy, a disease which makes it harder for heart to pump blood to the entire body, and heart-rate disorders.
The advisory mentions that HCQ, in rare cases, can cause visual disturbance, including “blurring of vision, which is usually self-limiting and improves on discontinuation of the drug”.
ICMR has clarified that “for the above cited reasons — heart and vision — the drug has to be given under strict medical supervision with an informed consent”.
Once again I doubt HCQ is some all encompassing solve all case drug given all the data – BUT to exclude it for a physician choices of treating their patient in some combination is highly concerning to me. The risk is very limited considering cost as part of the risk equation vs. a drug who is 1000X the cost and has 21% serious adverse reaction plus shown to only help in reducing timing – mortality reduction is not statistically valid… Things that make you go hmmmmmm
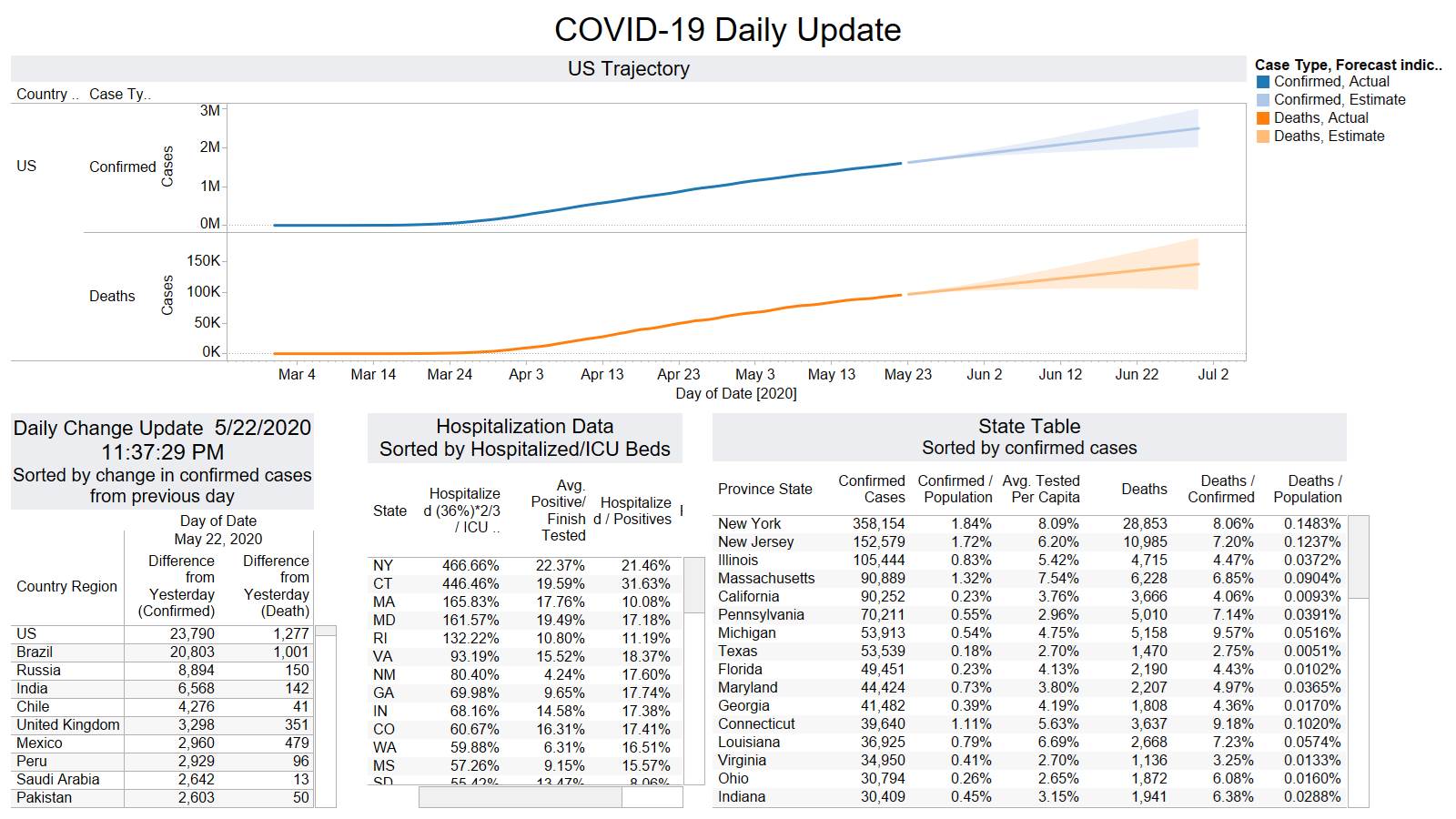
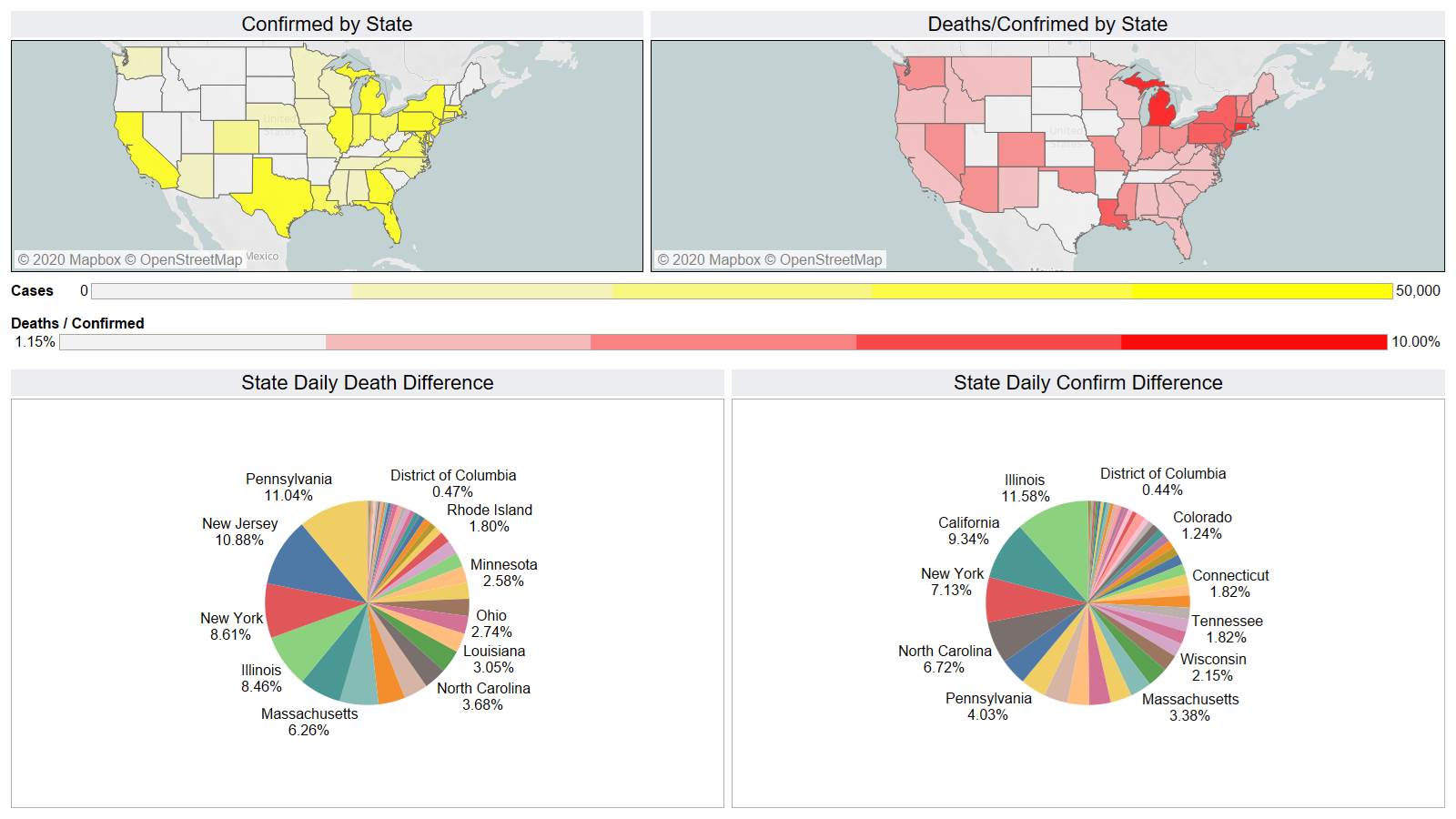
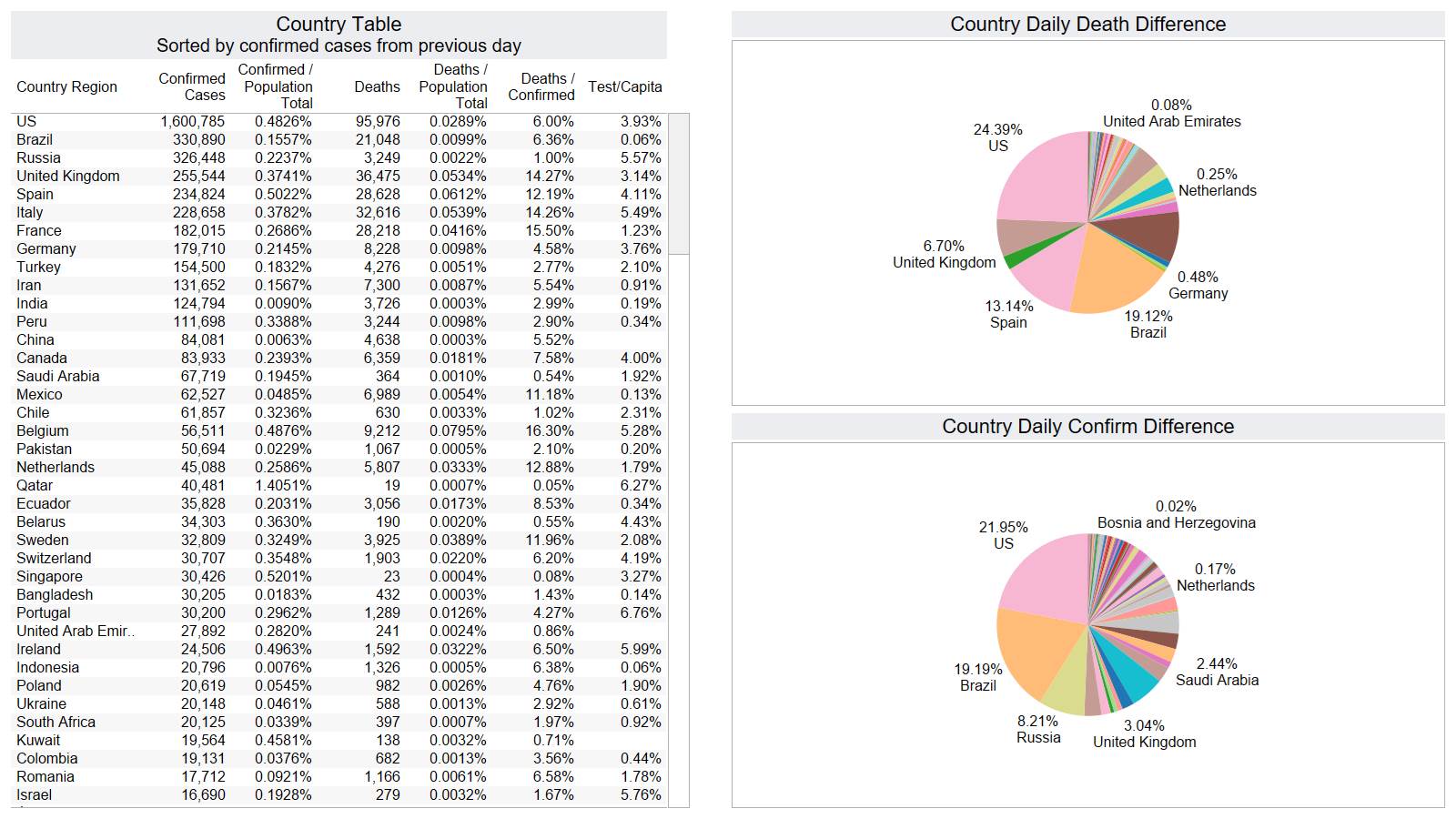
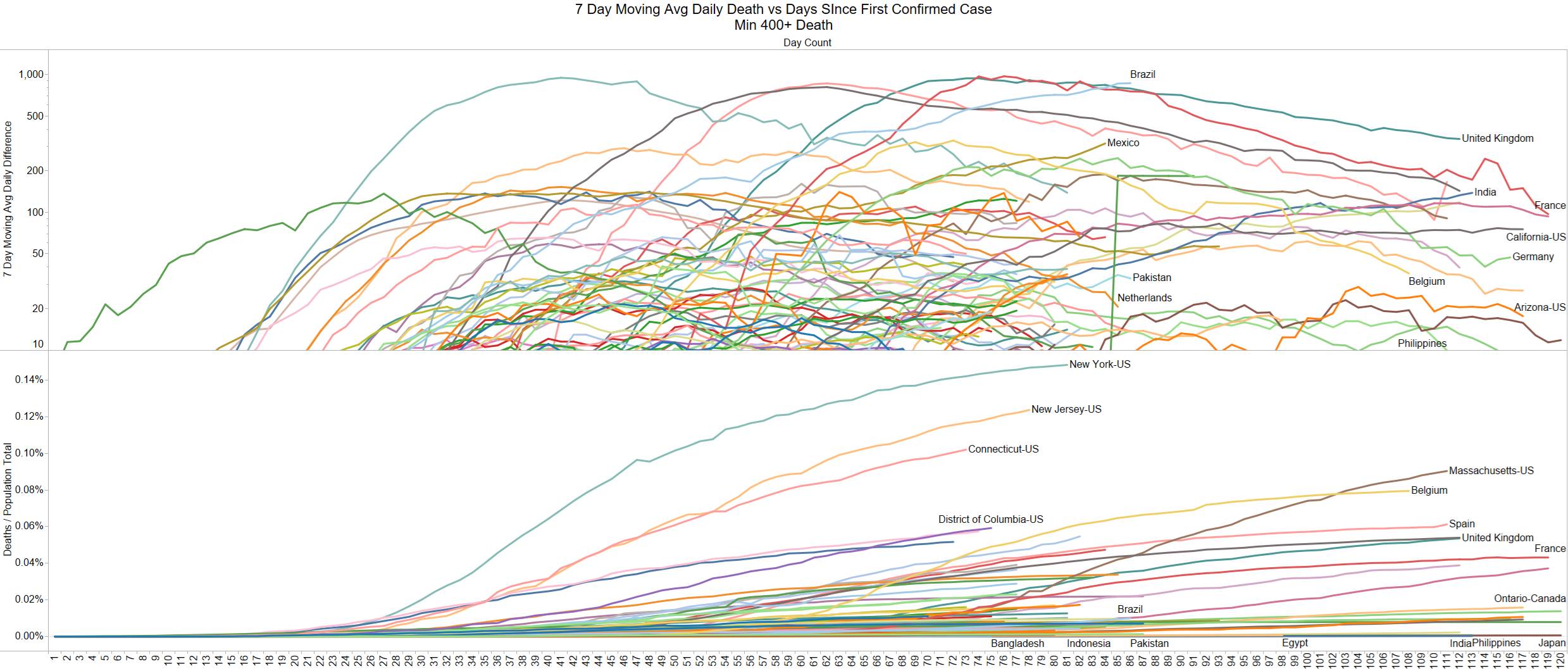
US 1277 – Brazil 1001 – they better start doing something there it is not looking good for them.
PA (141) took the lead from NY (139)
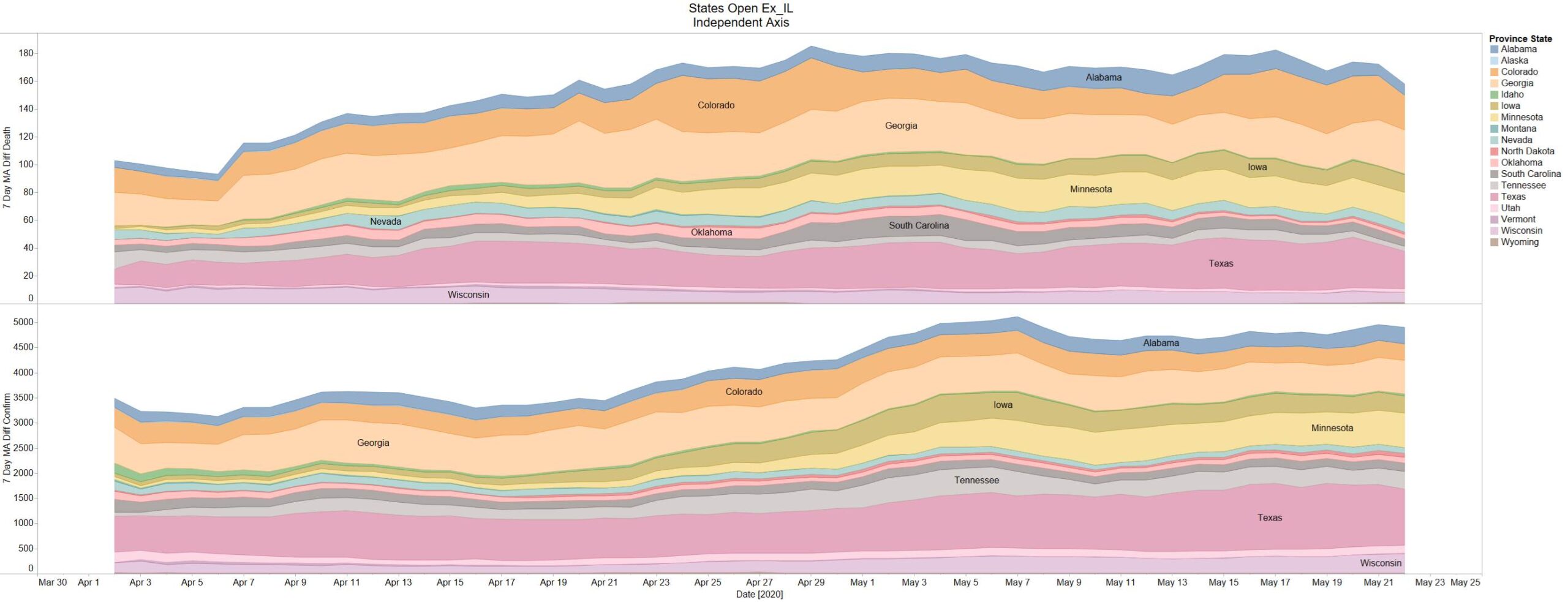
So far nothing to report in the opened states in May – so far looks fine.
Hoping the testing data for Brazil is stale.
Brazil has surpassed Spain 7 day MA daily death peak. France is taking a big dive down.