The big news going around is a cheap drug that can improve survival – dexamethasone. Hate to be a downer here but there are some important points not really highlighted in many articles. First we discussed the use of steroids back 5/12 in the must watch testimony in the morning report https://covid19mathblog.com/2020/05/covid-5-12-20/ when Dr. Kory testified Pierre Kory, M.D., M.P.A.
Critical Care Service Chief
Associate Professor of Medicine
University of Wisconsin School of Medicine and Public Health
https://www.hsgac.senate.gov/imo/media/doc/Testimony-Kory-2020-05-06-REVISED.pdf
He was using Methylprednisolone for treatment WHEN ADMITTED TO HOSPITAL. So IF you are at this point things are not looking too good for you. This solution is a late stage solution. This is not helping you not to get to this point. The other concern about this is the source of the paper where this comes from https://apnews.com/89d963958b042cc921e64ab3eff5a74d :
“The Oxford study is the same one that earlier this month showed the malaria drug hydroxychloroquine was not working against the coronavirus. The study enrolled more than 11,000 patients in England, Scotland, Wales and Northern Ireland who were given either standard care or that plus one of several treatments: dexamethasone; hydroxychloroquine; the HIV combo drug lopinavir-ritonavir; the antibiotic azithromycin; the anti-inflammatory drug tocilizumab; or plasma from people who have recovered from COVID-19 that contains antibodies to fight the virus. Research is continuing on the other treatments. The research is funded by government health agencies in the United Kingdom and private donors including the Bill and Melinda Gates Foundation. ”
It would seem they used dosage of HCQ 6X what most other places have used in their study. This is a noted concern and they have not released results or response to this inquiry.
It looks like Brazil is joining the India alliance to use HCQ vs. what the FDA and WHO would like – https://amp.cnn.com/cnn/2020/06/16/americas/brazil-hydroxychloroquine-recommendations-fda-intl/index.html?
Pretty sad when the Brazil Health Ministry is criticizing FDA methods.
“Research around the world subsequently cast doubt on its effectiveness, and the FDA ultimately determined that the drugs do not meet "the statutory criteria" for emergency use authorization
But Pinheiro on Tuesday dismissed the studies cited by the FDA, claiming that the "quality of their methodology is terrible."
She also said, without presenting any evidence, that coronavirus cases in Brazil had gone down since May 20, when the Health Ministry approved new guidelines allowing the use of chloroquine and hydroxychloroquine for all patients displaying mild, moderate and severe Covid-19 symptoms if both doctor and patient agree on the use of the drugs.”
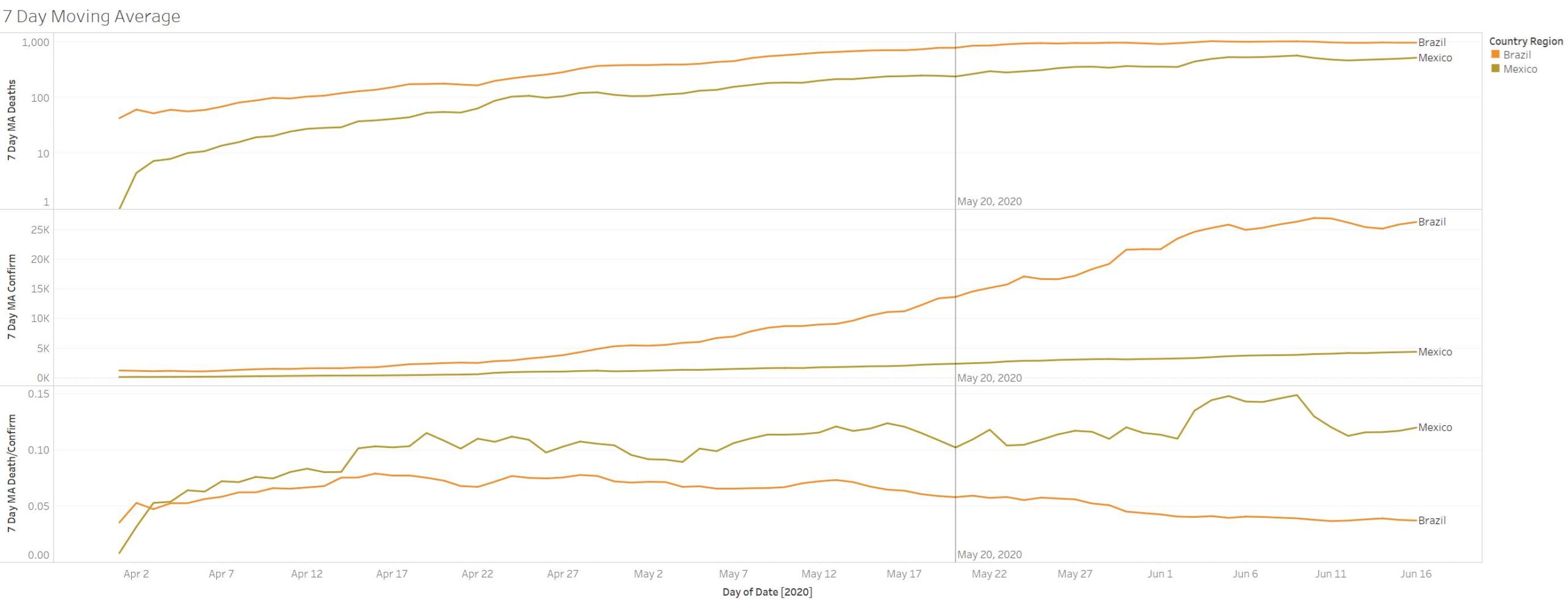
Ok so here is some evidence – which a journalist in CNN could have done – IF what she says is true they started May 20th – then fatality rates should have dropped – which they did. Remember this drug treatment is NOT going to stop the spread- so death will rise as confirmation rise – what needs to happen IF the drug is doing what it needs to do the fatality rates should drop – and this happens to be the case. Now there was a slight trend a week before May 20th before the drop but the trajectory has maintained itself. Unlike Mexico where they are not using HCQ they have not observed any drop in fatality rate.
WSJ is catching up – the report pretty much summarizes our morning report using the same reports I highlighted to come to the conclusions and proposed guidelines– https://www.wsj.com/articles/how-exactly-do-you-catch-covid-19-there-is-a-growing-consensus-11592317650?
“Health agencies have so far identified respiratory-droplet contact as the major mode of Covid-19 transmission. These large fluid droplets can transfer virus from one person to another if they land on the eyes, nose or mouth. But they tend to fall to the ground or on other surfaces pretty quickly.
Some researchers say the new coronavirus can also be transmitted through aerosols, or minuscule droplets that float in the air longer than large droplets. These aerosols can be directly inhaled.”
“Sufficient ventilation in the places people visit and work is very important, said Yuguo Li, one of the authors and an engineering professor at the University of Hong Kong. Proper ventilation—such as forcing air toward the ceiling and pumping it outside, or bringing fresh air into a room—dilutes the amount of virus in a space, lowering the risk of infection.”
“Another factor is prolonged exposure. That’s generally defined as 15 minutes or more of unprotected contact with someone less than 6 feet away, said John Brooks, the Centers for Disease Control and Prevention’s chief medical officer for the Covid-19 response. But that is only a rule of thumb, he cautioned. It could take much less time with a sneeze in the face or other intimate contact where a lot of respiratory droplets are emitted, he said.”
“Being outside is generally safer, experts say, because viral particles dilute more quickly. But small and large droplets pose a risk even outdoors, when people are in close, prolonged contact, said Linsey Marr, a Virginia Tech environmental engineering professor who studies airborne transmission of viruses.
No one knows for sure how much virus it takes for someone to become infected, but recent studies offer some clues. In one small study published recently in the journal Nature, researchers were unable to culture live coronavirus if a patient’s throat swab or milliliter of sputum contained less than one million copies of viral RNA.”
“CDC guidelines for employers whose workers are returning include requiring masks, limiting use of public transit and elevators to reduce exposure, and prohibiting hugs, handshakes and fist-bumps. The agency also suggested replacing communal snacks, water coolers and coffee pots with prepacked, single-serve items, and erecting plastic partitions between desks closer than 6 feet apart”
“the bigger risks are close-range face-to-face interactions, and having lots of people in an enclosed space for long periods. High-touch surfaces like doorknobs are a risk, but the virus degrades quickly so other surfaces like cardboard boxes are less worrisome, he said. “Surfaces and cleaning are important, but we shouldn’t be spending half of our budget on it when they may be having only a smaller effect,” he said.”
“The places he worries about are the break rooms, locker rooms and security checkpoints, where people interact. Those are spaces where the company has instituted social-distancing measures by staggering the times they are open and how many people can be there at once. Only a few cafeterias are open, and those that are have socially distanced seating. In bathrooms, only half the stalls are available to cut down on the number of people.”
How one gets covid covered 5/15/20
https://covid19mathblog.com/2020/05/covid-5-15-20/
Suggested Guidelines 5/18/20
https://covid19mathblog.com/2020/05/covid-5-18-20/
Vaccine progressing in China – going to phase 3 – China got a head start I wouldn’t be surprise they get to a vaccine first.
“Inside one of the new production facilities the China National Biotec Group (CNBG) has built for manufacturing a Covid-19 vaccines CNBG announced that one of the potential vaccine candidates had completed phase one and phase two clinical trials. Photo: The State-owned Assets Supervision and Administration CommissionInside one of the new production facilities the China National Biotec Group (CNBG) has built for manufacturing a Covid-19 vaccines CNBG announced that one of the potential vaccine candidates had completed phase one and phase two clinical trials. Photo: The State-owned Assets Supervision and Administration Commission
Inside one of the new production facilities the China National Biotec Group (CNBG) has built for manufacturing a Covid-19 vaccines CNBG announced that one of the potential vaccine candidates had completed phase one and phase two clinical trials. Photo: The State-owned Assets Supervision and Administration Commission
A potential Chinese Covid-19 vaccine, which has completed phase one and phase two human trials, is generally safe and can generate immune response in test subjects, the vaccine’s developer, China National Biotec Group, said on Tuesday.
The vaccine candidate, developed by a CNBG subsidiary, the Wuhan Institute of Biological Products, began human trials in Henan province in April. Volunteers 18 to 59 years old were inoculated with low, medium and high doses and were given a second shot two weeks, three weeks or four weeks later to study the safety and immunity response of the vaccine, CNBG said.”
“The programme inoculating volunteers with two doses four weeks apart induced neutralising antibodies – blocking pathogens from infecting human cells – in all its test subjects, the company said.
“This study is the world’s first clinical trial to obtain safety and effectiveness data of a two-dose inactivated Covid-19 vaccine … The research also involves the longest period, the most comprehensive data and the most satisfying clinical research results of Covid-19 vaccine clinical trial,” the group said. The company said that it was “actively advancing” the phase three clinical trial overseas and that it had reached “intention of cooperation” with companies and institutions in numerous countries. China is not considered suitable for the trial, which would involve at least thousands of volunteers, because the number of Covid-19 patients has dropped significantly there and it would be difficult to determine whether the vaccine could actually prevent a test subject from getting sick.””
“The company announced earlier that it has reached an agreement with Instituto Butantan in Brazil to prepare and conduct a phase three clinical study”
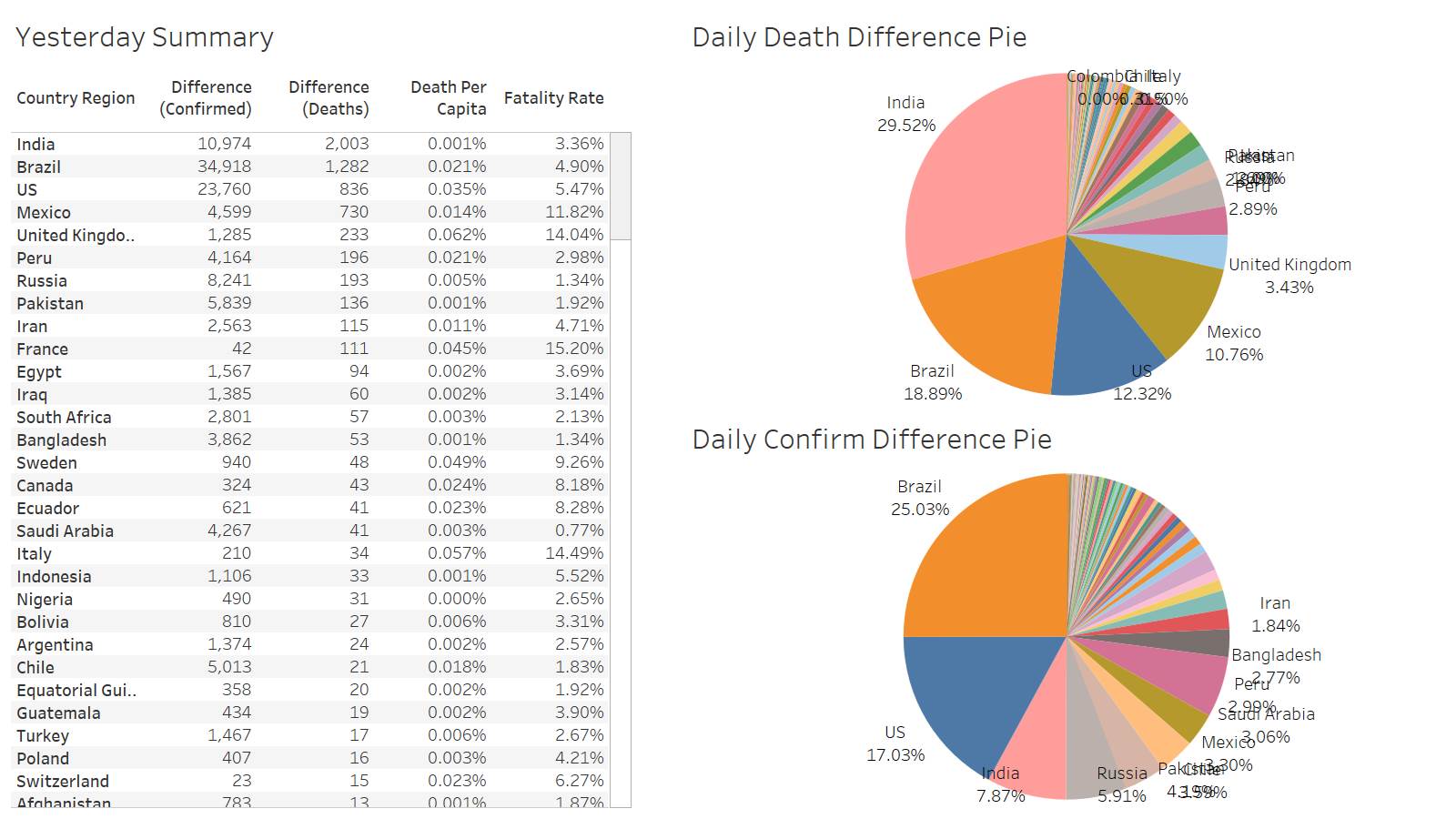
Not a good day for India 2003 deaths reported yesterday. Brazil 1282 US maintained below 1000 – 836
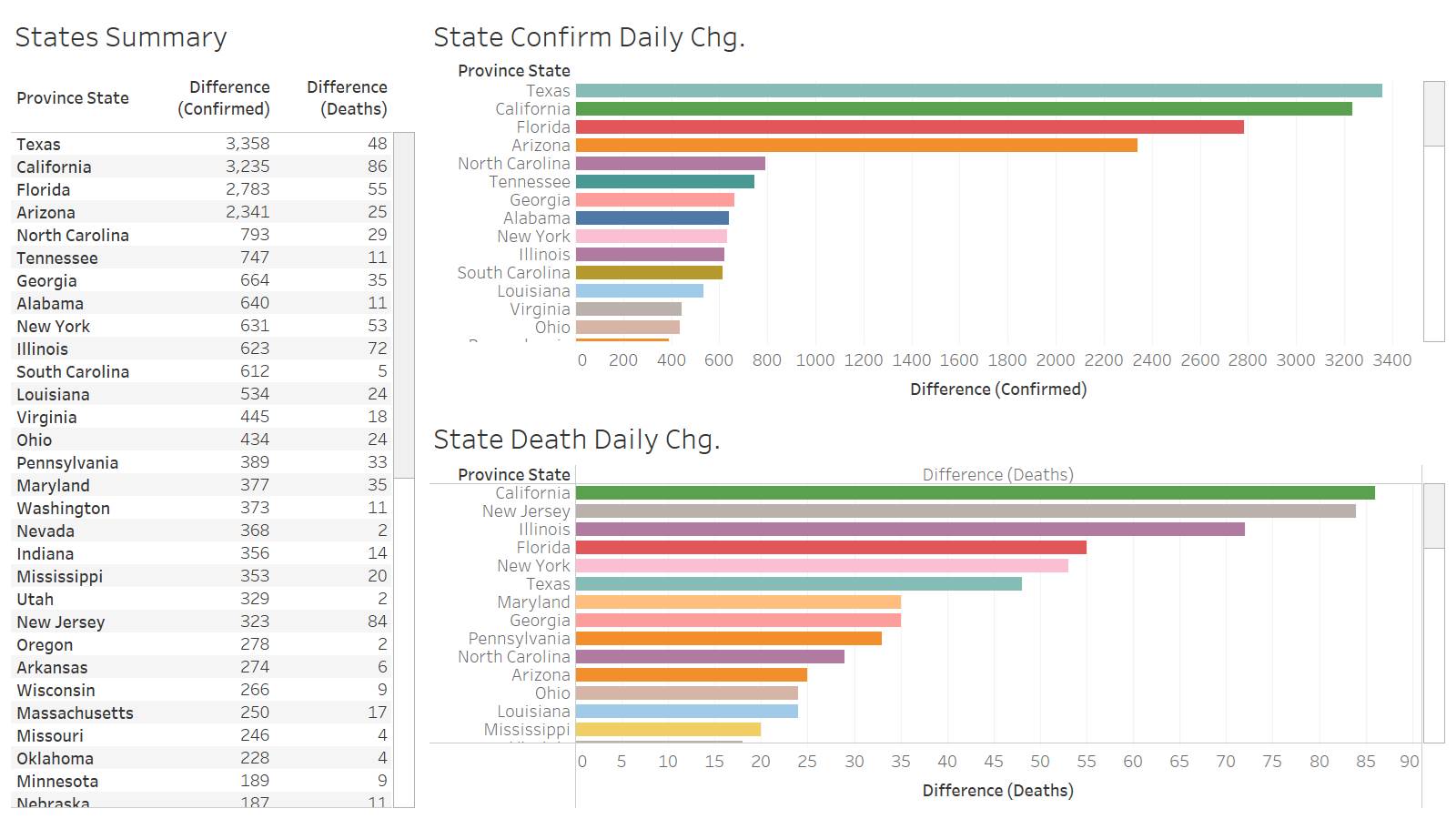
CA leads the way in US death at 86. The big story likely is TX big jump in confirmation at 3358.
TX confirmation seems to be wrong given Brazoria county confirmation jumped 613 in 1 day. That’s more than the total they have….Cant confirm with county data….
This caused the delay of this release….hopefully we see a fix in tomorrow data release. https://www.brazoriacountytx.gov/departments/health-department/brazoria-county-coronavirus-map
Or at least an explanation. I know the difference in one area is the counting of the correctional facilities.
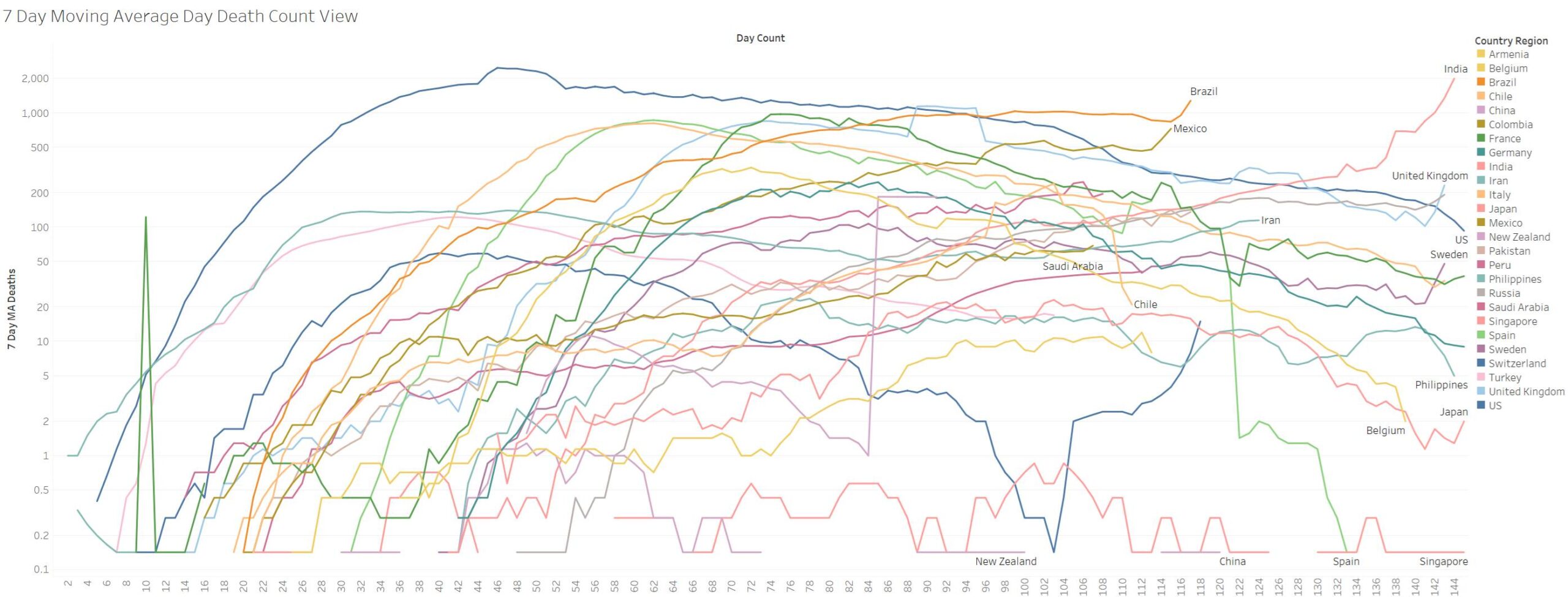
Not good for India and Brazil and Mexico, Russia, UK, Sweden all surging in deaths